2021 Updates to the Outpatient Management COPD: Prevent Inpatient Management of COPD
Dr. Jeff Spindel
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of death worldwide and is estimated to cost 49 billion dollars annually for treatment and missed work in America.¹ Since COPD is common, preventable, and treatable, this blog post will discuss the outpatient management of stable COPD and recent updates to the guidelines.
First, some definitions are necessary.
COPD Classifications:
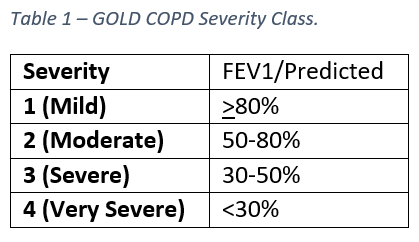
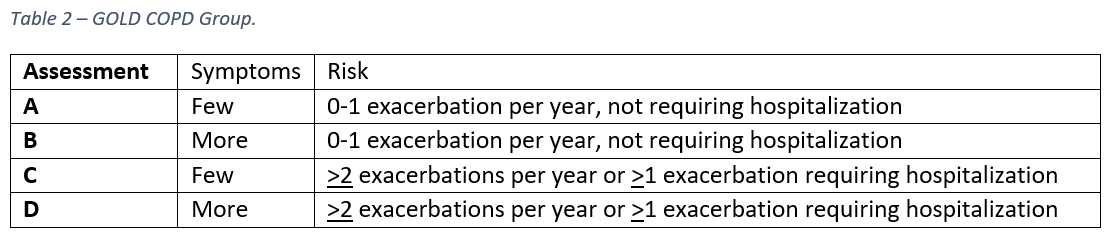
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria classifies patients into both severity class, based on airflow limitation (table 1), and group, based on assessment of symptoms and risk of hospitalization (table 2). Airflow limitation is determined via spirometry and symptom assessment is performed using either a Modified MRC Dyspnea Scale (mMRC) or a COPD Assessment Test (CAT).
COPD Treatment:
Medications for COPD are either targeted at dilating the small airway musculature, like beta agonists or muscarinic antagonists, or they target inflammation, like inhaled or systemic corticosteroids.
Treatment for COPD is based on symptom class and hospitalization risk in table ². Initial therapy should consist of a long-acting beta agonist (LABA) or long-acting muscarinic antagonist (LAMA) for a patient in any group (A-D) and escalation as needed to LABA and LAMA. If further escalation is warranted and blood eosinophil counts are >300 cells/microliter, an inhaled corticosteroid (ICS) can also be added.²,³
In the 2018 guidelines, the addition of an inhaled corticosteroid (ICS) was recommended for patients with COPD group C and D.2 Now in 2021, an ICS is not recommended unless patients have group D COPD or an elevated peripheral eosinophil count. Before going into the details, it is important to note that the backbone of treatment for asthma remains ICS, and these recommendations were upheld in the 2021 guidelines published by the Global Initiative for Asthma (GINA).³
Reasons for these changes to the COPD treatment guidelines are multiple:
1. ICS are beneficial only in select circumstances.⁴–⁶
Recent data suggests a clinical benefit from ICS only when used in conjunction with both LABA and LAMA.
Current smokers and past heavy smokers do not benefit as much from ICS use as do their counterparts.
There is almost no benefit to lung function or risk of exacerbations if eosinophil counts are <100 cells/microliter.
In patients with elevated blood eosinophil counts (>300), beneficial effects of ICS are only on reducing exacerbations, not mortality.
2. Use of ICS is associated with multiple, serious side effects, especially when used long term (>3 years).⁷–¹³
Regular treatment with ICS increase the risk of pneumonia, especially in patients with severe COPD.
ICS can cause worse glycemic control, cataracts, and mycobacterial infection.
ICS treatment and lung cancer risk have conflicting results.
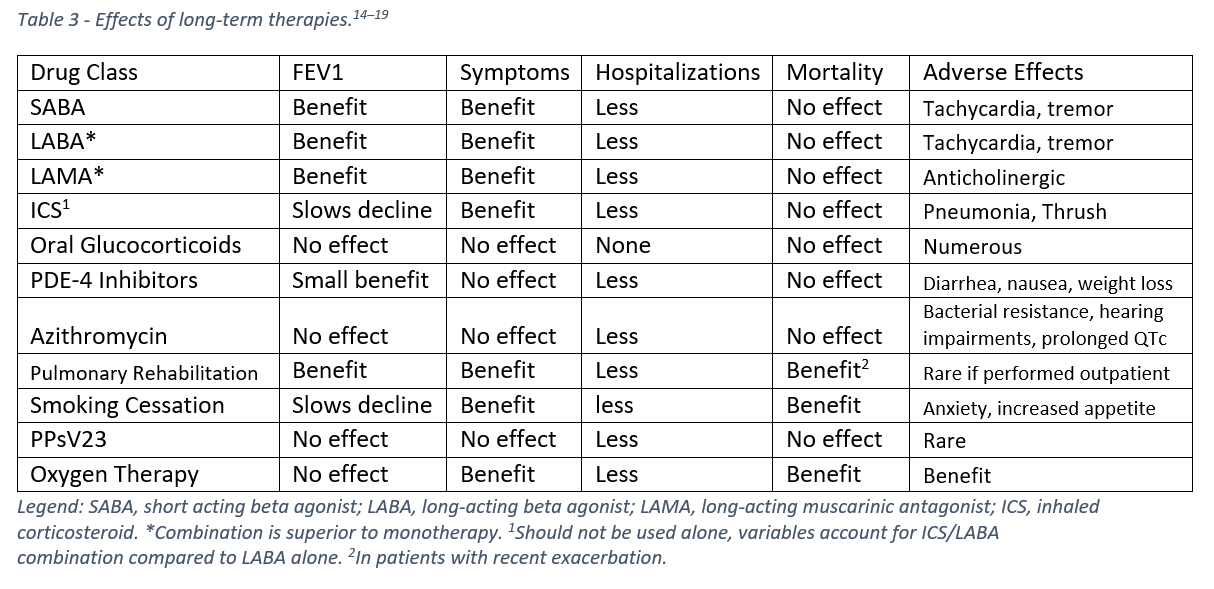
What hasn’t changed on the 2021 GOLD Guidelines is a primary goal for the outpatient management of COPD – reducing exacerbations. This is because inhalers and other medications don’t reduce mortality. Only smoking cessation, pulmonary rehabilitation, and oxygen (when indicated) have been shown to reduce mortality.¹⁴
Logically, medications that reduce exacerbations should reduce mortality, but data conflicts and only specific subgroups have significant mortality benefits from individual therapies.¹⁴ Because the largest proportion of cost from COPD is attributed to hospitalizations, therapies can be effective if they reduce hospitalizations even if there is no improvement to mortality or lung function.¹⁴ Table 3 summarizes the effects of current treatment options for COPD.
Apart from medications, smoking cessation counselling and intervention should be attempted frequently and continued until sustained abstinence is achieved. Counselling has been shown to be effective, and a strong dose-response relationship exists between intensity and frequency of counselling and smoking cessation.¹⁴
In summary:
Stratify patients based on symptoms and risk of hospitalizations.
Target therapies for reduction in symptoms and/or a reduction in hospitalizations.
Counsel smoking cessation whenever possible.
ICS are beneficial only in select circumstances.
Use of ICS is associated with multiple, serious side effects, especially when used long term.
Only smoking cessation, pulmonary rehabilitation, and oxygen therapy reduce mortality from COPD.
Additional considerations:
Inhalers won’t work if not used properly.
Severe spirometry does not necessarily predict frequency of exacerbations or need for hospitalization.²⁰
Past hospitalizations predict future hospitalizations.¹⁴
Jeff Spindel, D.O.
University of Louisville | UL · Department of Medicine | Doctor of Osteopathic Medicine
Jeffrey Spindel is currently a resident in Internal Medicine at the University of Louisville. His current research and academic interests include the effects of blood glucose control on the incidence of major adverse cardiac events and utilization of low cost coronary artery calcium scoring for risk stratification in asymptomatic patients.
References:
COPD Costs. Published July 5, 2019. Accessed November 6, 2021. https://www.cdc.gov/copd/infographics/copd-costs.html
2018 Global Initiative for Chronic Obstructive Lung Disease. GOLD. Published 2018. Accessed November 24, 2021. https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf
Global Strategy for Asthma Management and Prevention. Published 2021. Accessed November 24, 2021. https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf
Lipson DA, Barnhart F, Brealey N, et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N Engl J Med. 2018;378(18):1671-1680. doi:10.1056/NEJMoa1713901
Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020;10(4):e037509. doi:10.1136/bmjopen-2020-037509
Singh D, Brooks J, Hagan G, Cahn A, O’Connor BJ. Superiority of “triple” therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax. 2008;63(7):592-598. doi:10.1136/thx.2007.087213
Lin SH, Perng DW, Chen CP, et al. Increased risk of community-acquired pneumonia in COPD patients with comorbid cardiovascular disease. Int J Chron Obstruct Pulmon Dis. 2016;11:3051-3058. doi:10.2147/COPD.S115137
Tashkin DP, Pearle J, Iezzoni D, Varghese ST. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD. 2009;6(1):17-25. doi:10.1080/1541255090272407
Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med. 2010;123(11):1001-1006. doi:10.1016/j.amjmed.2010.06.019
Suissa S, Dell’Aniello S, Gonzalez AV, Ernst P. Inhaled corticosteroid use and the incidence of lung cancer in COPD. European Respiratory Journal. 2020;55(2). doi:10.1183/13993003.01720-2019
Wang JJ, Rochtchina E, Tan AG, Cumming RG, Leeder SR, Mitchell P. Use of inhaled and oral corticosteroids and the long-term risk of cataract. Ophthalmology. 2009;116(4):652-657. doi:10.1016/j.ophtha.2008.12.001
Andréjak C, Nielsen R, Thomsen VØ, Duhaut P, Sørensen HT, Thomsen RW. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68(3):256-262. doi:10.1136/thoraxjnl-2012-201772
Price D, Yawn B, Brusselle G, Rossi A. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim Care Respir J. 2013;22(1):92-100. doi:10.4104/pcrj.2012.00092
2021 GOLD Reports. Global Initiative for Chronic Obstructive Lung Disease - GOLD. Accessed November 6, 2021. https://goldcopd.org/2021-gold-reports
Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med. 2014;189(12):1503-1508. doi:10.1164/rccm.201402-0207OC
Talman S, Uzun S, Djamin RS, et al. <p>Long-Term Azithromycin Maintenance Treatment in Patients with Frequent Exacerbations of Chronic Obstructive Pulmonary Disease</p>. COPD. 2021;16:495-498. doi:10.2147/COPD.S284397
Simmons MS, Connett JE, Nides MA, et al. Smoking reduction and the rate of decline in FEV1: results from the Lung Health Study. European Respiratory Journal. 2005;25(6):1011-1017. doi:10.1183/09031936.05.00086804
Pavlov N, Haynes AG, Stucki A, Jüni P, Ott SR. Long-term oxygen therapy in COPD patients: population-based cohort study on mortality. Int J Chron Obstruct Pulmon Dis. 2018;13:979-988. doi:10.2147/COPD.S154749
Ringbaek TJ, Viskum K, Lange P. Does long-term oxygen therapy reduce hospitalisation in hypoxaemic chronic obstructive pulmonary disease? Eur Respir J. 2002;20(1):38-42. doi:10.1183/09031936.02.00284202
Han MK, Quibrera PM, Carretta EE, et al. Frequency of Exacerbations in COPD: An Analysis of the SPIROMICS Cohort. Lancet Respir Med. 2017;5(8):619-626. doi:10.1016/S2213-2600(17)30207-2